About
The most recent data reveal:
- Nearly 1 in 50 people in the United States lives with paralysis, making up almost 5.4 million people or 1.7% of the US population.
- Leading causes of paralysis, in order of incidence rate, are stroke (33.7%), spinal cord injury (27.3%), and multiple sclerosis (18.6%).
- Only 15.5% of individuals living with paralysis are employed, and 41.8% of individuals with paralysis report that they are unable to work.
- Causes of spinal cord injury, in order of incidence are motor vehicle accidents, physical labor, falls, sports accidents, and victims of violence.
- Spinal cord injury alone cost the country roughly $40.5 billion per year, a figure that has increased 317% since 1998.
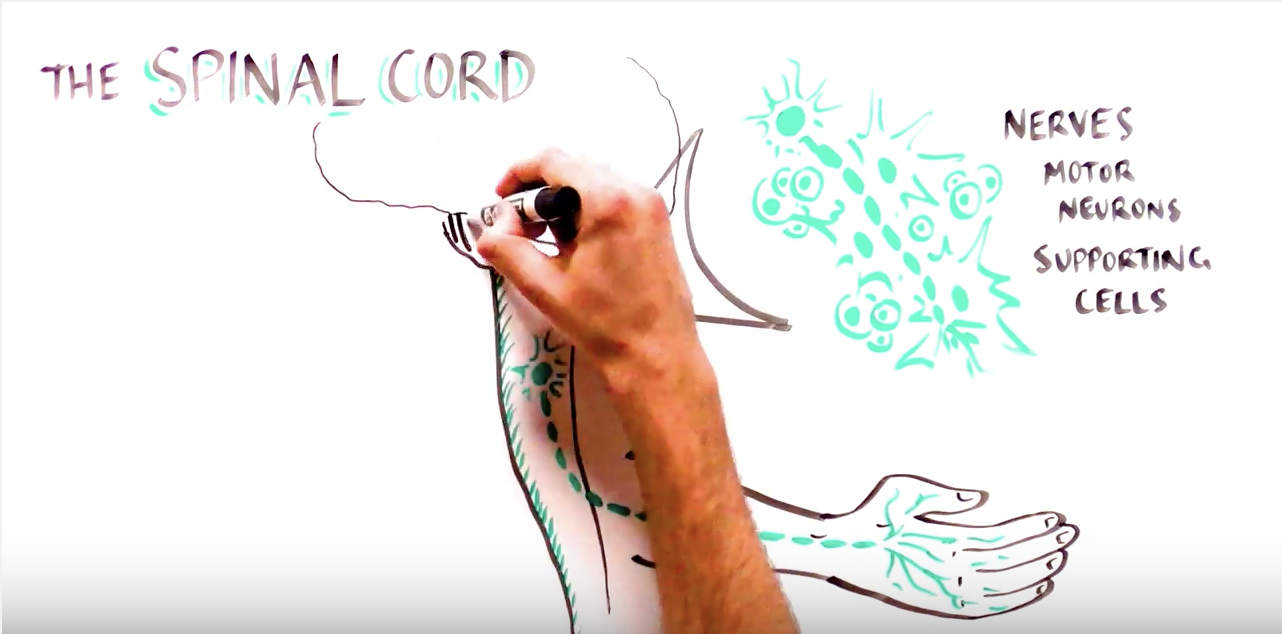
How Stem Cell Research Can be Used to Treat Paralysis
The world’s first clinical trial using human embryonic stem cell-derived populations targeted spinal cord injury. Much of the research in the area of stem cell transplantation focuses on identification of the most appropriate population for either cell replacement or the protection of existing cells.
CIRM’s Progress: Selected Research Highlights
- CIRM has funded three clinical trials that affect paralysis:
- One in stroke
- Two in spinal cord injury
- In a Phase 1/2 clinical trial, researchers at Lineage Cell Therapeutics use stem cells to generate oligo progenitors — the cells that protect nerves by keeping them insulated and ready for signal transmission. Notably, 96% of the patients enrolled in this trial have shown at least one level of motor recovery at 12 months post-transplantation.
- In a Phase 2 clinical trial, researchers at SanBio isolated mesenchymal stem cells from the bone marrow of healthy adult donors, modified them to secrete “food” for existing nerve cells in the brain, and transplanted them after stroke. While the transplantation was shown to be safe, this study did not meet its primary endpoints. However, ongoing work using the same cell population may be leading to Phase 3 clinical trials in Traumatic Brain Injury.
Brain and Spinal Cord Organizations Endorsing YES on Prop 14
- Spinal Cord Injury International